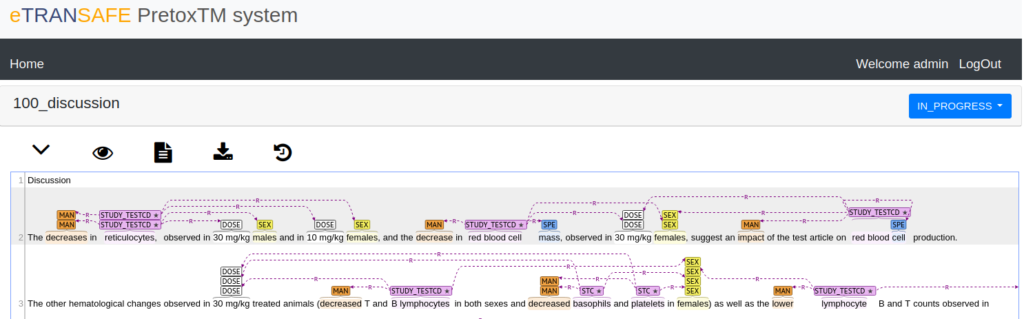
A new version of the eTRANSAFE preclinical text mining system, PretoxTM 2.0, was presented in a virtual workshop on the 22nd of November 2021 by members of the project Work Package 5.
The workshop was organized as a brainstorming session to obtain end-user feedback from toxicology experts. The insights gathered during the event will be used to feed the continuous improvements of the preclinical text mining system and will be incorporated in subsequent versions.
(more…)