Version 10 of the SR-Domain Specification and v1.1.0 of the SR-Domain Editor, developed by SME Partner PDS Consultants (PDS), were released in December 2020 for use by the EFPIA partners of eTRANSAFE.
The SR-Domain template is based on the SEND standard and uses the CDISC SEND Controlled Terminology (CT) for compatibility with associated SEND datasets. The SR-Domain defines all the key entities that describe a treatment-related finding, obtained from the Summary section of study reports, and is designed to be integrated with the eTRANSAFE pre-clinical database.
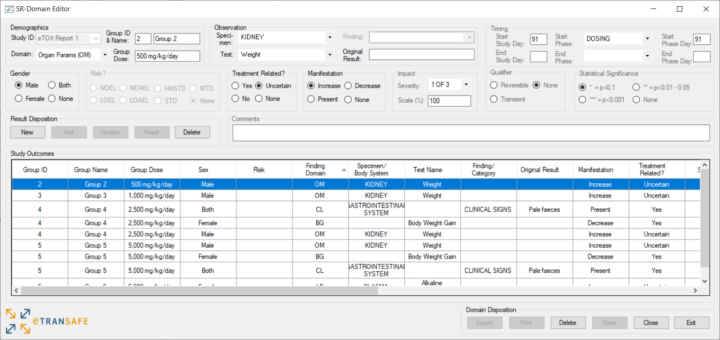
The SR-Domain Editor is a desktop application which enables SR-Domain templates to be visualised, amended and added to. It can also be used to create SR-Domains from scratch as well as being the target for holding Text Mining results.
The SR-Domain concept is raising wide interest both within and outside the consortium. It has been shared with several external stakeholders comprising an industry-wide audience of CROs, Sponsors and Regulators.
