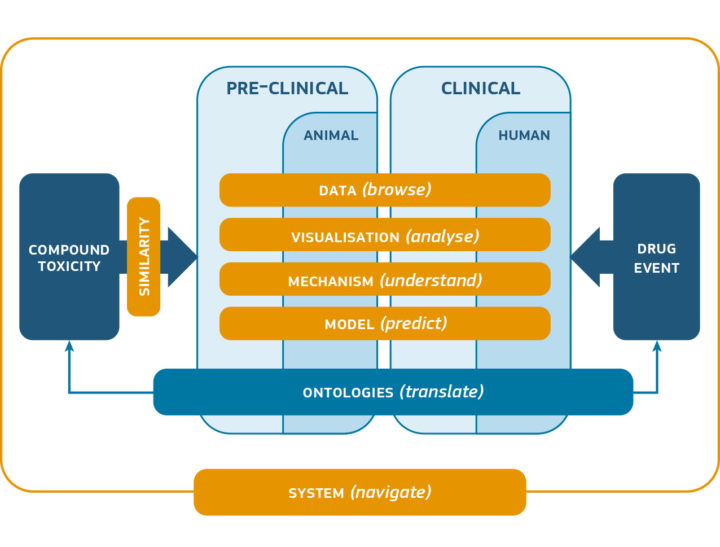
- The development of a state-of-the-art, powerful and flexible strategy and technological architecture for data sharing (diverse sources of nonclinical and clinical data), data integration and data exploitation.
- The implementation of the following key elements for this architecture: a specialised SEND (“standards for the exchange of non-clinical data”) data management system, a database of shared proprietary data managed by an honest broker, a Knowledge Hub providing seamless access to all the databases and data sources, and an ecosystem of data exploitation modules.
- The establishment of overarching policies and guidelines for safeguarded data sharing, secondary use of human safety data and use of pooled data and models in drug safety assessment.
- Optimising how preclinical studies are run and how the industry designs these studies.
